
CHEBI:16027
| Name | adenosine 5'-monophosphate | 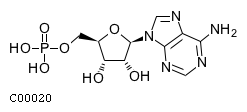 Download: mol | sdf |
| Synonyms | 05'-adenosine monophosphate; 5'-adenylic acid; 5'-amp; 5'-o-phosphonoadenosine; 5'-o-phosphonatoadenosine; Adenosine 5'-(dihydrogen phosphate); Adenosine 5'-monophosphate; Adenosine 5'-phosphate; Adenosine monophosphate; Adenosine phosphate; Adenosine-5'p; Adenylate; Adenylic acid; Ado5'p; Amp; Pado; | |
| Definition | A purine ribonucleoside 5'-monophosphate having adenine as the nucleobase. | |
| Molecular Weight (Exact mass) | 347.2212 (347.0631) | |
| Molecular Formula | C10H14N5O7P | |
| SMILES | Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O | |
| InChI | InChI=1S/C10H14N5O7P/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(17)6(16)4(22-10)1-21-23(18,19)20/h2-4,6-7,10,16-17H,1H2,(H2,11,12,13)(H2,18,19,20)/t4-,6-,7-,10-/m1/s1 | |
| InChI Key | UDMBCSSLTHHNCD-KQYNXXCUSA-N | |
| Crosslinking annotations | KEGG:C00020 | 3DMET:B01133 | CAS:61-19-8 | ChEBI:16027 | ChEMBL:CHEMBL752 | KNApSAcK:C00019347 | NIKKAJI:J4.814C | PDB-CCD:A | PDB-CCD:AMP | PDB-CCD:AP7 | PubChem:3322 | |
| Pathway ID | Pathway Name | Pathway Description (KEGG) |
| map00230 | Purine metabolism | NA |
| map00908 | Zeatin biosynthesis | Zeatin is a member of the cytokinin family, a class of phytohormones involved in various processes of growth and development in plants. Most abundant cytokinins are adenine-type, where the N6 position of adenine is substituted with an isoprenoid, such as in zeatin, or an aromatic side chain, such as in kinetin. Zeatin can be synthesized in two different pathways: the tRNA pathway and the AMP pathway. In the tRNA pathway zeatin is a recycled product of isopentenylated tRNAs. In the AMP pathway zeatin is synthesized from an isopentenyl donor, dimethylallyl diphosphate (DMAPP), and AMP, ADP, or ATP by isopentenyltransferases. After synthesis cytokinins can be glucosylated. |
| map01060 | Biosynthesis of plant secondary metabolites | NA |
| map01065 | Biosynthesis of alkaloids derived from histidine and purine | NA |
| map01070 | Biosynthesis of plant hormones | NA |
| map01100 | Metabolic pathways | NA |
| map01110 | Biosynthesis of secondary metabolites | NA |
| map01130 | Biosynthesis of antibiotics | NA |
| map01523 | Antifolate resistance | Since the 1940s, antifolates have played a pivotal role in drug treatment of malignant, microbial, parasitic and chronic inflammatory diseases. The molecular basis of the anti-proliferative activity of antifolates relies on inhibition of key enzymes in folate metabolism, which results in disruption of purine and thymidylate biosynthesis, inhibition of DNA replication and cell death. The anti-inflammatory properties of antifolate have been most strongly related to its ability to block the release of pro-inflammatory cytokines such as tumour necrosis factor (TNF)-alpha or interleukin (IL)-1beta. Cells may develop resistance to an antifolate drug by virtue of impaired drug transport into cells, augmented drug export, impaired activation of antifolates through polyglutamylation, augmented hydrolysis of antifolate polyglutamates, increased expression and mutation of target enzymes, and the augmentation of cellular tetrahydrofolate-cofactor pools in cells. |
| map04022 | cGMP-PKG signaling pathway | Cyclic GMP (cGMP) is the intracellular second messenger that mediates the action of nitric oxide (NO) and natriuretic peptides (NPs), regulating a broad array of physiologic processes. The elevated intracellular cGMP level exerts its physiological action through two forms of cGMP-dependent protein kinase (PKG), cGMP-regulated phosphodiesterases (PDE2, PDE3) and cGMP-gated cation channels, among which PKGs might be the primary mediator. PKG1 isoform-specific activation of established substrates leads to reduction of cytosolic calcium concentration and/or decrease in the sensitivity of myofilaments to Ca2+ (Ca2+-desensitization), resulting in smooth muscle relaxation. In cardiac myocyte, PKG directly phosphorylates a member of the transient potential receptor canonical channel family, TRPC6, suppressing this nonselective ion channel's Ca2+ conductance, G-alpha-q agonist-induced NFAT activation, and myocyte hypertrophic responses. PKG also opens mitochondrial ATP-sensitive K+ (mitoKATP) channels and subsequent release of ROS triggers cardioprotection. |
| map04024 | cAMP signaling pathway | cAMP is one of the most common and universal second messengers, and its formation is promoted by adenylyl cyclase (AC) activation after ligation of G protein-coupled receptors (GPCRs) by ligands including hormones, neurotransmitters, and other signaling molecules. cAMP regulates pivotal physiologic processes including metabolism, secretion, calcium homeostasis, muscle contraction, cell fate, and gene transcription. cAMP acts directly on three main targets: protein kinase A (PKA), the exchange protein activated by cAMP (Epac), and cyclic nucleotide-gated ion channels (CNGCs). PKA modulates, via phosphorylation, a number of cellular substrates, including transcription factors, ion channels, transporters, exchangers, intracellular Ca2+ -handling proteins, and the contractile machinery. Epac proteins function as guanine nucleotide exchange factors (GEFs) for both Rap1 and Rap2. Various effector proteins, including adaptor proteins implicated in modulation of the actin cytoskeleton, regulators of G proteins of the Rho family, and phospholipases, relay signaling downstream from Rap. |
| map04068 | FoxO signaling pathway | The forkhead box O (FOXO) family of transcription factors regulates the expression of genes in cellular physiological events including apoptosis, cell-cycle control, glucose metabolism, oxidative stress resistance, and longevity. A central regulatory mechanism of FOXO proteins is phosphorylation by the serine-threonine kinase Akt/protein kinase B (Akt/PKB), downstream of phosphatidylinositol 3-kinase (PI3K), in response to insulin or several growth factors. Phosphorylation at three conserved residues results in the export of FOXO proteins from the nucleus to the cytoplasm, thereby decreasing expression of FOXO target genes. In contrast, the stress-activated c-Jun N-terminal kinase (JNK) and the energy sensing AMP-activated protein kinase (AMPK), upon oxidative and nutrient stress stimuli phosphorylate and activate FoxOs. Aside from PKB, JNK and AMPK, FOXOs are regulated by multiple players through several post-translational modifications, including phosphorylation, but also acetylation, methylation and ubiquitylation. |
| map04150 | mTOR signaling pathway | The mammalian (mechanistic) target of rapamycin (mTOR) is a highly conserved serine/threonine protein kinase, which exists in two complexes termed mTOR complex 1 (mTORC1) and 2 (mTORC2). mTORC1 contains mTOR, Raptor, PRAS40, Deptor, mLST8, Tel2 and Tti1. mTORC1 is activated by the presence of growth factors, amino acids, energy status, stress and oxygen levels to regulate several biological processes, including lipid metabolism, autophagy, protein synthesis and ribosome biogenesis. On the other hand, mTORC2, which consists of mTOR, mSin1, Rictor, Protor, Deptor, mLST8, Tel2 and Tti1, responds to growth factors and controls cytoskeletal organization, metabolism and survival. |
| map04151 | PI3K-Akt signaling pathway | The phosphatidylinositol 3' -kinase(PI3K)-Akt signaling pathway is activated by many types of cellular stimuli or toxic insults and regulates fundamental cellular functions such as transcription, translation, proliferation, growth, and survival. The binding of growth factors to their receptor tyrosine kinase (RTK) or G protein-coupled receptors (GPCR) stimulates class Ia and Ib PI3K isoforms, respectively. PI3K catalyzes the production of phosphatidylinositol-3,4,5-triphosphate (PIP3) at the cell membrane. PIP3 in turn serves as a second messenger that helps to activate Akt. Once active, Akt can control key cellular processes by phosphorylating substrates involved in apoptosis, protein synthesis, metabolism, and cell cycle. |
| map04152 | AMPK signaling pathway | AMP-activated protein kinase (AMPK) is a serine threonine kinase that is highly conserved through evolution. AMPK system acts as a sensor of cellular energy status. It is activated by increases in the cellular AMP:ATP ratio caused by metabolic stresses that either interfere with ATP production (eg, deprivation for glucose or oxygen) or that accelerate ATP consumption (eg, muscle contraction). Several upstream kinases, including liver kinase B1 (LKB1), calcium/calmodulin kinase kinase-beta (CaMKK beta), and TGF-beta-activated kinase-1 (TAK-1), can activate AMPK by phosphorylating a threonine residue on its catalytic alpha-subunit. Once activated, AMPK leads to a concomitant inhibition of energy-consuming biosynthetic pathways, such as protein, fatty acid and glycogen synthesis, and activation of ATP-producing catabolic pathways, such as fatty acid oxidation and glycolysis. |
| map04211 | Longevity regulating pathway | Regulation of longevity depends on genetic and environmental factors. Caloric restriction (CR), that is limiting food intake, is recognized in mammals as the best characterized and most reproducible strategy for extending lifespan. Four pathways have been implicated in mediating the CR effect. These are the insulin like growth factor (IGF-1)/insulin signaling pathway, the sirtuin pathway, the adenosine monophosphate (AMP) activated protein kinase (AMPK) pathway and the target of rapamycin (TOR) pathway. The collective response of these pathways to CR is believed to promote cellular fitness and ultimately longevity via activation of autophagy, stress defense mechanisms, and survival pathways while attenuating proinflammatory mediators and cellular growth. Furthermore, there is evidence supporting that life span extension can be achieved with pharmacologic agents that mimic the effects of caloric restriction, such as rapamycin, via mTOR signaling blockade, resveratrol, by activating SIRT1 activity, and metformin, which seems to be a robust stimulator of AMPK activity. As an aging suppressor, Klotho is an important molecule in aging processes and its overexpression results in longevity. |
| map04740 | Olfactory transduction | Within the compact cilia of the olfactory receptor neurons (ORNs) a cascade of enzymatic activity transduces the binding of an odorant molecule to a receptor into an electrical signal that can be transmitted to the brain. Odorant molecules bind to a receptor protein (R) coupled to an olfactory specific Gs-protein (G) and activate a type III adenylyl cyclase (AC), increasing intracellular cAMP levels. cAMP targets an olfactory-specific cyclic-nucleotide gated ion channel (CNG), allowing cations, particularly Na and Ca, to flow down their electrochemical gradients into the cell, depolarizing the ORN. Furthermore, the Ca entering the cell is able to activate a Ca-activated Cl channel, which would allow Cl to flow out of the cell, thus further increasing the depolarization. Elevated intracellular Ca causes adaptation by at least two different molecular steps: inhibition of the activity of adenylyl cyclase via CAMKII-dependent phosphorylation and down-regulation of the affinity of the CNG channel to cAMP. |
| map04742 | Taste transduction | Five basic tastes are recognized by humans and most other animals - bitter, sweet, sour, salty and umami. In vertebrates, taste stimuli are detected by taste receptor cells (TRCs). At least three distinct cell types are found in mammalian taste buds : type I cells, type II cells, and type III cells. Type I cells express epithelial sodium channel (ENaC) and are considered to be the major mediator of perception of low salt. In type II cells, transduction of bitter, sweet and umami is mediated by a canonical PLC-beta/IP3-signaling cascade, which culminates in the opening of the TRPM5 ion channel. This produces a depolarization that may allow CALMH1 channels to open and release ATP, which serves as a neurotransmitter to activate closely associated nerve afferents expressing P2X2, P2X3 receptors and adjacent type III cells expressing P2Y4 receptors. Type II taste cells also secrete acetylcholine (ACh) that appears to stimulate muscarinic receptors, specifically M3, on the same or neighboring Type II cells. This muscarinic feedback augments taste-evoked release of ATP. In type III cells, sour taste is initiated when protons enter through apically located proton-selective ion channels: polycystic kidney disease 2-like 1 protein (PKD2L1) and polycystic kidney disease 1-like 3 protein (PKD1L3) channels. Weak acids may also activate sour cells by penetrating the cell membrane and leading to closure of resting K+ channels and membrane depolarization. Further, voltage-gated Ca2+ channels are activated and release vesicular serotonin (5-HT), norepinephrine (NE) and gamma-aminobutyric acid (GABA). 5-HT and GABA provide negative paracrine feedback onto receptor cells by activating 5-HT1A and GABAA, GABAB receptors, respectively. 5-HT also functions as a transmitter between presynaptic cells and the sensory afferent. |
| map04923 | Regulation of lipolysis in adipocytes | Lipolysis in adipocytes, the hydrolysis of triacylglycerol (TAG) to release fatty acids (FAs) and glycerol for use by other organs as energy substrates, is a unique function of white adipose tissue. Lipolysis is under tight hormonal control. During fasting, catecholamines, by binding to Gs-coupled-adrenergic receptors (-AR), activate adenylate cyclase (AC) to increase cAMP and activate protein kinase A (PKA). PKA phosphorylates target protein such as hormone-sensitive lipase (HSL) and perilipin 1 (PLIN). PLIN phosphorylation is a key event in the sequential activation of TAG hydrolysis involving adipose triglyceride lipase (ATGL), HSL, and monoglyceride lipase (MGL). During the fed state, insulin, through activation of phosphodiesterase-3B (PDE-3B), inhibits catecholamine-induced lipolysis via the degradation of cAMP. |
| map04924 | Renin secretion | The aspartyl-protease renin is the key regulator of the renin-angiotensin-aldosterone system, which is critically involved in extracellular fluid volume and blood pressure homeostasis of the body. Renin is synthesized, stored in, and released into circulation by the juxtaglomerular (JG) cells of the kidney. Secretion of renin from JG cells at the organ level is controlled by the four main mechanisms: the sympathetic nervous system, the local JG apparatus baroreflex, the macula densa mechanism, and several hormones acting locally within the JG apparatus. Renin secretion at the level of renal JG cells appears to be controlled mainly by classic second messengers, namely cAMP, cGMP, and free cytosolic calcium concentration. While cAMP generally stimulates renin release and the intracellular calcium concentration suppresses the exocytosis of renin, the effects of cGMP in the regulation of the renin system are more complex as it both may stimulate or inhibit renin release. |
| map04925 | Aldosterone synthesis and secretion | Aldosterone is a steroid hormone synthesized in and secreted from the outer layer of the adrenal cortex, the zona glomerulosa. Aldosterone plays an important role in the regulation of systemic blood pressure through the absorption of sodium and water. Angiotensin II (Ang II), potassium (K+) and adrenocorticotropin (ACTH) are the main extracellular stimuli which regulate aldosterone secretion. These physiological agonists all converge on two major intracellular signaling pathways: calcium (Ca2+) mobilization and an increase in cAMP production. The increase in cytosolic calcium levels activates calcium/calmodulin- dependent protein kinases (CaMK), and the increased cAMP levels stimulate the activity of cAMP-dependent protein kinase, or protein kinase A (PKA). The activated CaMK, and possibly PKA, activates transcription factors (NURR1 and NGF1B, CREB) to induce StAR and CYP11B2 expression, the early and late rate- limiting steps in aldosterone biosynthesis, respectively, thereby stimulating aldosterone secretion. |
| map04927 | Cortisol synthesis and secretion | Cortisol is the main endogenous glucocorticoid, which affects a plethora of physiological functions, e.g., lipid and glucose metabolism, metabolic homeostasis and adaptation to stress. Cortisol production is primarily regulated by corticotropin (ACTH) in zona fasciculata. The stimulatory effect of ACTH on cortisol synthesis depends on cAMP dependent signaling, but also involves membrane depolarization and increased cytosolic Ca2+. Each of cAMP and Ca2+ induces the expression of StAR, stimulating intramitochondrial cholesterol transfer, as well as the steroidogenic enzymes in the pathway from cholesterol to cortisol (e.g., CHE, CYP17A1, CYP11B1). |
| map04928 | Parathyroid hormone synthesis, secretion and action | Parathyroid hormone (PTH) is a key regulator of calcium and phosphorus homeostasis. The principal regulators of PTH secretion are extracellular ionized calcium (Ca2+) and 1,25-dihydroxyvitamin D (1,25(OH)2D3). Under conditions of dietary Ca restriction, a decrement in serum Ca concentration induces release of PTH from the parathyroid gland. PTH acts on bone and kidney to stimulate bone turnover, increase the circulating levels of 1,25(OH)2D3 and calcium and inhibit the reabsorption of phosphate from the glomerular filtrate. This hormone exerts its actions via binding to the PTH/PTH-related peptide receptor (PTH1R). PTH1R primarily activates two sub-types of heterotrimeric Gproteins: Gs and Gq , which in turn regulate the activity of adenylyl cyclases and phospholipase C (PLC) that control the flow of cAMP/PKA and IP/PKC signaling cascades, respectively. |
| map04934 | Cushing syndrome | Cushing syndrome (CS) is a rare disorder resulting from prolonged exposure to excess glucocorticoids via exogenous and endogenous sources. The typical clinical features of CS are related to hypercortisolism and include accumulation of central fat, moon facies, neuromuscular weakness, osteoporosis or bone fractures, metabolic complications, and mood changes. Traditionally, endogenous CS is classified as adrenocorticotropic hormone (ACTH)-dependent (about 80%) or ACTH- independent (about 20%). Among ACTH-dependent forms, pituitary corticotroph adenoma (Cushing's disease) is most common. Most pituitary tumors are sporadic, resulting from monoclonal expansion of a single mutated cell. Recently recurrent activating somatic driver mutations in the ubiquitin-specific protease 8 gene (USP8) were identified in almost half of corticotroph adenoma. Germline mutations in MEN1 (encoding menin), AIP (encoding aryl-hydrocarbon receptor-interacting protein), PRKAR1A (encoding cAMP-dependent protein kinase type I alpha regulatory subunit) and CDKN1B (encoding cyclin-dependent kinase inhibitor 1B; also known as p27 Kip1) have been identified in familial forms of pituitary adenomas. However, the frequency of familial pituitary adenomas is less than 5% in patients with pituitary adenomas. Among ACTH-independent CS, adrenal adenoma is most common. Rare adrenal causes of CS include primary bilateral macronodular adrenal hyperplasia (BMAH) or primary pigmented nodular adrenocortical disease (PPNAD). |
| map05012 | Parkinson disease | Parkinson disease (PD) is a progressive neurodegenerative movement disorder that results primarily from the death of dopaminergic (DA) neurons in the substantia nigra pars compacta (SNc). Mutations in alpha-synuclein, UCHL1 (a ubiquitin carboxy-terminal hydrolase L1), parkin, DJ1 (a parkin-associated protein involved with oxidative stress), and PINK1 (a putative serine threonine kinase) are known to cause early-onset PD. Mutations or altered expression of these proteins contributes to the damage and subsequent loss of DA neurons through common mechanisms that result in proteasome dysfunction, mitochondrial impairment, and oxidative stress. The demise of DA neurons located in the SNc leads to a drop in the dopaminergic input to the striatum. This results in a reduced activation of the direct pathway and in a disinhibition of the indirect pathway, which is associated with the elevation of adenosine A2A receptor transmission. Such unbalanced activity of the striatal output pathway is at the basis of the motor impairment observed in PD. |
| map05032 | Morphine addiction | Morphine is an alkaloid from the plant extracts of opium poppy. Although morphine is highly effective for the treatment of pain, it is also known to be intensely addictive. We now know that the most important brain-reward circuit involves dopamine (DA) -containing neurons in the ventral tegmental area (VTA) of the midbrain and their target areas in the limbic forebrain, in particular, the nucleus accumbens (NAc) and frontal regions of cerebral cortex. Morphine can cause indirect excitation of VTA dopamine neurons by reducing inhibitory synaptic transmission mediated by GABAergic neurons. The chronic use of morphine is characterized by adaptive changes in neurons and neuronal communication; such adaptations (e.g., 'superactivation' of adenylyl cyclase) must underlie altered behaviour associated with morphine dependence and withdrawal syndrome, as well as drug-induced craving and relapse to drug use. |

